-
Light-Driven Functionalization of Unreactive Sites Using Oxidative Amination
LIGHT-N-RING
Funded by:

Supported by:

Project details
doc. dr. sc. Davor Šakić
HRZZ project UIP 2020-02-4857
February 1, 2021 – January 31, 2026
Project budget: 2 000 000,00 kn
Location: University of Zagreb,
Faculty of Pharmacy and Biochemistry
-
-
-
Background
C-H functionalization methods are often used in late-stage functionalization synthesis in the pharmaceutical industry to generate new compounds from a lead compound. These reactions utilize unreactive sp3 C-H bonds, providing a way for exploring the chemical space more efficiently than conventional synthesis. Novel C(sp3)-H functionalization reactions with chemo-, regio-, and stereo-specificity, combined with easily prepared precursors under mild conditions are actively sought by numerous groups. The search for amination reactions of the C-H bond in metal-free conditions has recently led to a resurgence in studies of the Hofmann-Löffler-Freytag (HLF) reaction and its modern variants. The current reaction sequence involves the generation of a labile N-halogen bond, followed by homolytic cleavage that produces unstable N-centered radicals, that undergo rearrangement reactions to the corresponding, more stable C-centered radicals. Formation of the C-halogen bond completes the radical chain process, while subsequent cyclisation occurs in a non-radical step.
Aims and objectives
In this project, the mechanism of metal-free C-H bond amination reactions will be explored to discover necessary chemical switches and/or reaction conditions to further increase the chemo-, regio-, stereo-specificity and the utility of these reactions. The goal is to quantify the effects of various electron-withdrawing groups on the N-centered radical (in)stability, which directly relates to the driving force of the reaction and uses this data for the rational design of HLF reactions. Competition between intra- and inter-molecular pathways must be analysed in more detail in order to offer a better understanding of the regioselectivity of the reaction. The underlying reaction mechanism needs a detailed re-evaluation after demonstrating that some reaction variants proceed through ionic mechanisms to products with intact stereochemical information. A detailed search for a chemical switch that governs which mechanism is active in selected reactions will be conducted.
Expected outcomes
• Clear understanding of role of substituents in driving force of the reaction
• Role of solvent molecules on reaction mechanism
• Discovery of chemical switches that govern the reaction outcome
• Installation of a new research group specialized in photo-organo-synthesis
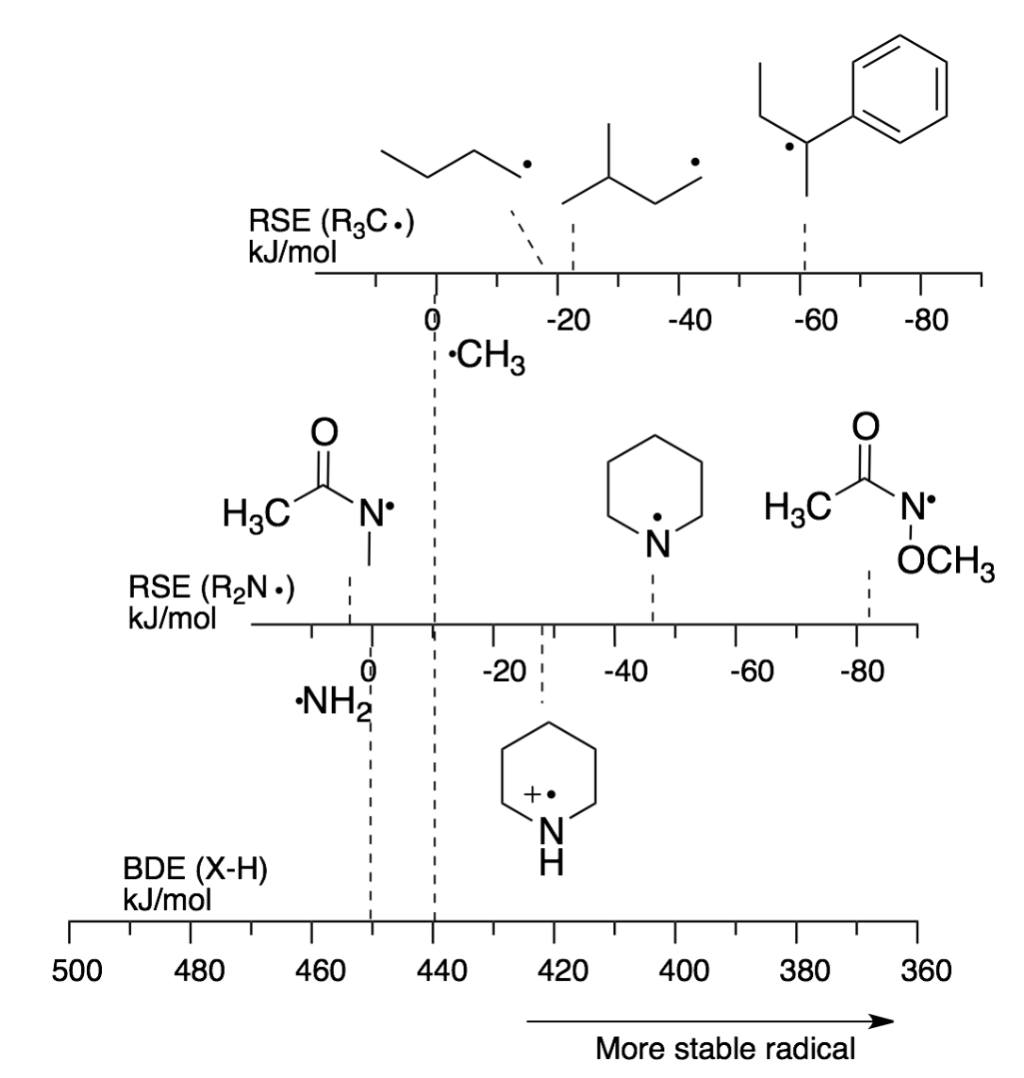
Figure 1: Radical stabilization energies (RSE) for selected radicals ploted in respect to the bond dissociation energies (BDE)
-